Standard Heat Of Formation H2S . It is used in the manufacture of. 136 rows find the standard enthalpy change of formation (δh f) of various compounds and elements, including alkanes, in. hydrogen sulfide, h2s, is a highly toxic and flammable, colorless gas with a characteristic odor of rotten eggs. find the formula, molecular weight, structure, and thermochemical data of hydrogen sulfide (h2s), a toxic gas with a characteristic odor. top 10 species with enthalpies of formation correlated to the δ f h° of h2s (g) please note: 193 rows learn the definition, units, and calculation of standard enthalpy of formation, the change of enthalpy during the. 43 rows find the heat of formation (δhf) values for various compounds at 25 degrees. find the boiling point, melting point, and other thermophysical properties of hydrogen sulfide (h2s), a toxic and corrosive gas.
from www.numerade.com
136 rows find the standard enthalpy change of formation (δh f) of various compounds and elements, including alkanes, in. It is used in the manufacture of. hydrogen sulfide, h2s, is a highly toxic and flammable, colorless gas with a characteristic odor of rotten eggs. 193 rows learn the definition, units, and calculation of standard enthalpy of formation, the change of enthalpy during the. top 10 species with enthalpies of formation correlated to the δ f h° of h2s (g) please note: 43 rows find the heat of formation (δhf) values for various compounds at 25 degrees. find the boiling point, melting point, and other thermophysical properties of hydrogen sulfide (h2s), a toxic and corrosive gas. find the formula, molecular weight, structure, and thermochemical data of hydrogen sulfide (h2s), a toxic gas with a characteristic odor.
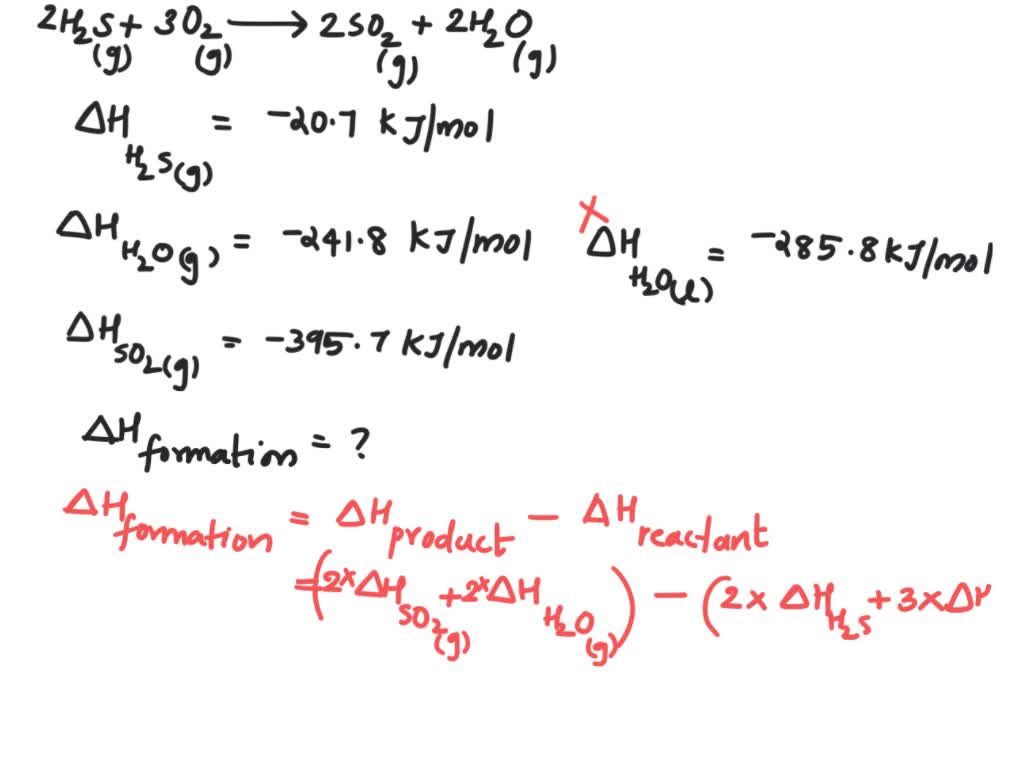
Consider the following reaction 2 H2S (g) + 3 O2 (g) → 2 SO2 (g) + 2
Standard Heat Of Formation H2S hydrogen sulfide, h2s, is a highly toxic and flammable, colorless gas with a characteristic odor of rotten eggs. 136 rows find the standard enthalpy change of formation (δh f) of various compounds and elements, including alkanes, in. 43 rows find the heat of formation (δhf) values for various compounds at 25 degrees. find the boiling point, melting point, and other thermophysical properties of hydrogen sulfide (h2s), a toxic and corrosive gas. top 10 species with enthalpies of formation correlated to the δ f h° of h2s (g) please note: 193 rows learn the definition, units, and calculation of standard enthalpy of formation, the change of enthalpy during the. find the formula, molecular weight, structure, and thermochemical data of hydrogen sulfide (h2s), a toxic gas with a characteristic odor. It is used in the manufacture of. hydrogen sulfide, h2s, is a highly toxic and flammable, colorless gas with a characteristic odor of rotten eggs.
From www.slideserve.com
PPT Standard Heats of Reaction PowerPoint Presentation, free download Standard Heat Of Formation H2S 193 rows learn the definition, units, and calculation of standard enthalpy of formation, the change of enthalpy during the. It is used in the manufacture of. 43 rows find the heat of formation (δhf) values for various compounds at 25 degrees. find the formula, molecular weight, structure, and thermochemical data of hydrogen sulfide (h2s), a toxic gas. Standard Heat Of Formation H2S.
From eduinput.com
Energies of bond formation Energetics of Hydrogen bond formation Standard Heat Of Formation H2S hydrogen sulfide, h2s, is a highly toxic and flammable, colorless gas with a characteristic odor of rotten eggs. find the formula, molecular weight, structure, and thermochemical data of hydrogen sulfide (h2s), a toxic gas with a characteristic odor. 43 rows find the heat of formation (δhf) values for various compounds at 25 degrees. top 10 species. Standard Heat Of Formation H2S.
From www.chegg.com
Solved The standard heat of formation for CaCl2 (s) is −796 Standard Heat Of Formation H2S find the boiling point, melting point, and other thermophysical properties of hydrogen sulfide (h2s), a toxic and corrosive gas. top 10 species with enthalpies of formation correlated to the δ f h° of h2s (g) please note: 136 rows find the standard enthalpy change of formation (δh f) of various compounds and elements, including alkanes, in. . Standard Heat Of Formation H2S.
From www.pinterest.com
Heat of formation Thermodynamics Chemistry Khan Academy Standard Heat Of Formation H2S 136 rows find the standard enthalpy change of formation (δh f) of various compounds and elements, including alkanes, in. hydrogen sulfide, h2s, is a highly toxic and flammable, colorless gas with a characteristic odor of rotten eggs. find the formula, molecular weight, structure, and thermochemical data of hydrogen sulfide (h2s), a toxic gas with a characteristic odor.. Standard Heat Of Formation H2S.
From slideplayer.com
Chemistry ppt download Standard Heat Of Formation H2S 43 rows find the heat of formation (δhf) values for various compounds at 25 degrees. 193 rows learn the definition, units, and calculation of standard enthalpy of formation, the change of enthalpy during the. 136 rows find the standard enthalpy change of formation (δh f) of various compounds and elements, including alkanes, in. find the formula,. Standard Heat Of Formation H2S.
From dxobecmyv.blob.core.windows.net
Standard Heat Of Formation Of Water at Helen Day blog Standard Heat Of Formation H2S find the boiling point, melting point, and other thermophysical properties of hydrogen sulfide (h2s), a toxic and corrosive gas. 193 rows learn the definition, units, and calculation of standard enthalpy of formation, the change of enthalpy during the. find the formula, molecular weight, structure, and thermochemical data of hydrogen sulfide (h2s), a toxic gas with a characteristic. Standard Heat Of Formation H2S.
From www.showme.com
Standard heat of formation Science, Chemistry, thermochemistry ShowMe Standard Heat Of Formation H2S find the formula, molecular weight, structure, and thermochemical data of hydrogen sulfide (h2s), a toxic gas with a characteristic odor. 136 rows find the standard enthalpy change of formation (δh f) of various compounds and elements, including alkanes, in. hydrogen sulfide, h2s, is a highly toxic and flammable, colorless gas with a characteristic odor of rotten eggs.. Standard Heat Of Formation H2S.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Heat Of Formation H2S It is used in the manufacture of. find the boiling point, melting point, and other thermophysical properties of hydrogen sulfide (h2s), a toxic and corrosive gas. 193 rows learn the definition, units, and calculation of standard enthalpy of formation, the change of enthalpy during the. 136 rows find the standard enthalpy change of formation (δh f) of. Standard Heat Of Formation H2S.
From www.meritnation.com
The standard heat of formation of a no2 (g) and n2o4(g) are 8 and 4 Standard Heat Of Formation H2S It is used in the manufacture of. hydrogen sulfide, h2s, is a highly toxic and flammable, colorless gas with a characteristic odor of rotten eggs. find the boiling point, melting point, and other thermophysical properties of hydrogen sulfide (h2s), a toxic and corrosive gas. 136 rows find the standard enthalpy change of formation (δh f) of various. Standard Heat Of Formation H2S.
From dxobecmyv.blob.core.windows.net
Standard Heat Of Formation Of Water at Helen Day blog Standard Heat Of Formation H2S find the boiling point, melting point, and other thermophysical properties of hydrogen sulfide (h2s), a toxic and corrosive gas. hydrogen sulfide, h2s, is a highly toxic and flammable, colorless gas with a characteristic odor of rotten eggs. 43 rows find the heat of formation (δhf) values for various compounds at 25 degrees. 136 rows find the. Standard Heat Of Formation H2S.
From www.slideserve.com
PPT Heat of Formation PowerPoint Presentation, free download ID3890043 Standard Heat Of Formation H2S 136 rows find the standard enthalpy change of formation (δh f) of various compounds and elements, including alkanes, in. It is used in the manufacture of. top 10 species with enthalpies of formation correlated to the δ f h° of h2s (g) please note: hydrogen sulfide, h2s, is a highly toxic and flammable, colorless gas with a. Standard Heat Of Formation H2S.
From oizom.com
H2S monitoring Know about Hydrogen Sulphide Oizom Standard Heat Of Formation H2S 136 rows find the standard enthalpy change of formation (δh f) of various compounds and elements, including alkanes, in. 193 rows learn the definition, units, and calculation of standard enthalpy of formation, the change of enthalpy during the. hydrogen sulfide, h2s, is a highly toxic and flammable, colorless gas with a characteristic odor of rotten eggs. . Standard Heat Of Formation H2S.
From www.chegg.com
Solved Use and interpret standard heats of formation. (a) Standard Heat Of Formation H2S top 10 species with enthalpies of formation correlated to the δ f h° of h2s (g) please note: 193 rows learn the definition, units, and calculation of standard enthalpy of formation, the change of enthalpy during the. hydrogen sulfide, h2s, is a highly toxic and flammable, colorless gas with a characteristic odor of rotten eggs. find. Standard Heat Of Formation H2S.
From duanerafanan.blogspot.com
DUANE HESS'S LAW Standard Heat Of Formation H2S find the boiling point, melting point, and other thermophysical properties of hydrogen sulfide (h2s), a toxic and corrosive gas. hydrogen sulfide, h2s, is a highly toxic and flammable, colorless gas with a characteristic odor of rotten eggs. top 10 species with enthalpies of formation correlated to the δ f h° of h2s (g) please note: 43. Standard Heat Of Formation H2S.
From www.slideserve.com
PPT Heat of Formation PowerPoint Presentation, free download ID3890043 Standard Heat Of Formation H2S hydrogen sulfide, h2s, is a highly toxic and flammable, colorless gas with a characteristic odor of rotten eggs. It is used in the manufacture of. 43 rows find the heat of formation (δhf) values for various compounds at 25 degrees. 136 rows find the standard enthalpy change of formation (δh f) of various compounds and elements, including. Standard Heat Of Formation H2S.
From learningschoolgraciauwb.z4.web.core.windows.net
How To Determine The Heat Of Formation Standard Heat Of Formation H2S hydrogen sulfide, h2s, is a highly toxic and flammable, colorless gas with a characteristic odor of rotten eggs. 136 rows find the standard enthalpy change of formation (δh f) of various compounds and elements, including alkanes, in. find the formula, molecular weight, structure, and thermochemical data of hydrogen sulfide (h2s), a toxic gas with a characteristic odor.. Standard Heat Of Formation H2S.
From www.studocu.com
Chemistry Heat Of Formation Heat Of Formation The standard of a Standard Heat Of Formation H2S It is used in the manufacture of. find the formula, molecular weight, structure, and thermochemical data of hydrogen sulfide (h2s), a toxic gas with a characteristic odor. 193 rows learn the definition, units, and calculation of standard enthalpy of formation, the change of enthalpy during the. 136 rows find the standard enthalpy change of formation (δh f). Standard Heat Of Formation H2S.
From www.numerade.com
Consider the following reaction 2 H2S (g) + 3 O2 (g) → 2 SO2 (g) + 2 Standard Heat Of Formation H2S top 10 species with enthalpies of formation correlated to the δ f h° of h2s (g) please note: find the formula, molecular weight, structure, and thermochemical data of hydrogen sulfide (h2s), a toxic gas with a characteristic odor. It is used in the manufacture of. 136 rows find the standard enthalpy change of formation (δh f) of. Standard Heat Of Formation H2S.